We know light can do a lot of different things to matter. It can change the composition and structure of chemical compounds. How does light excite molecules? What can this excitation trigger? What is light bleaching and photodegradation? How could we possibly use these concepts in designing mechanisms for the ray cats?
Absorption and Reflection
These are two of the basic concepts of light and color. Absorption is when a photon is passing through a material, and excites or resonates with an electron. The electron absorbs the photon’s energy and moves it to a higher energy state. The light stops, and the material is opaque. Reflection is when the effect of the vibrations made by the photon is enough to vibrate the electron same amount but not enough to excite it. The electron can’t use the energy to do work so the photon is reemitted back, or reflected. This is why black shirts are hot, why mirrors fog up, and it’s also what determines color. We know light comes in many wavelengths or colors, as we most often call them. But here is where it gets tricky, and creates a common misconception. A lot of times we use “colors” to describe the wavelength we see being emitted, and, as kids, we soon connect color with paint. So, if I were to ask: if you were to mix all the colors together what would you get? Black, right? But that’s not showing what the wavelengths of color become after they’ve been combined. It’s showing what wavelengths the pigments in the paint were taking out, or absorbing. In the white light coming from the sun or light bulbs, there is all the colors combined, while black paint absorbs all colors. Paint absorbs all the colors of white light, except for the one that you see, which gets reflected. Red paint just absorbs all the wavelengths except red.
Excitation
When materials absorb light, it’s taking energy from light and using that energy for something else. Most of the time, matter isn’t doing much more than converting the light’s vibrational energy into thermal energy, or heat. But, a lot of different things can also occur depending on the material, or compound. Excitation is when they use this energy to take an electron from one energy state to the other. This can be an electrical excitation or a vibrational one. Electrical excitations are like the little sparks or bursts of energy that are used to change the electron’s energy state, usually releasing a photon and falling back into place. This is also what happens in fluorescence or phosphorescence, where the glowing that we see in the dark is the photons that get released by electrons which had previously absorbed energy from light.Vibrational excitation happens when the excited energy state of the electron is not caused by some little bump or zap, but instead by resonance which is a more constant energy change, allowing the whole atom to vibrate and produce/conduct heat. We are interested in compounds that take electrical excitations and use it to change color.
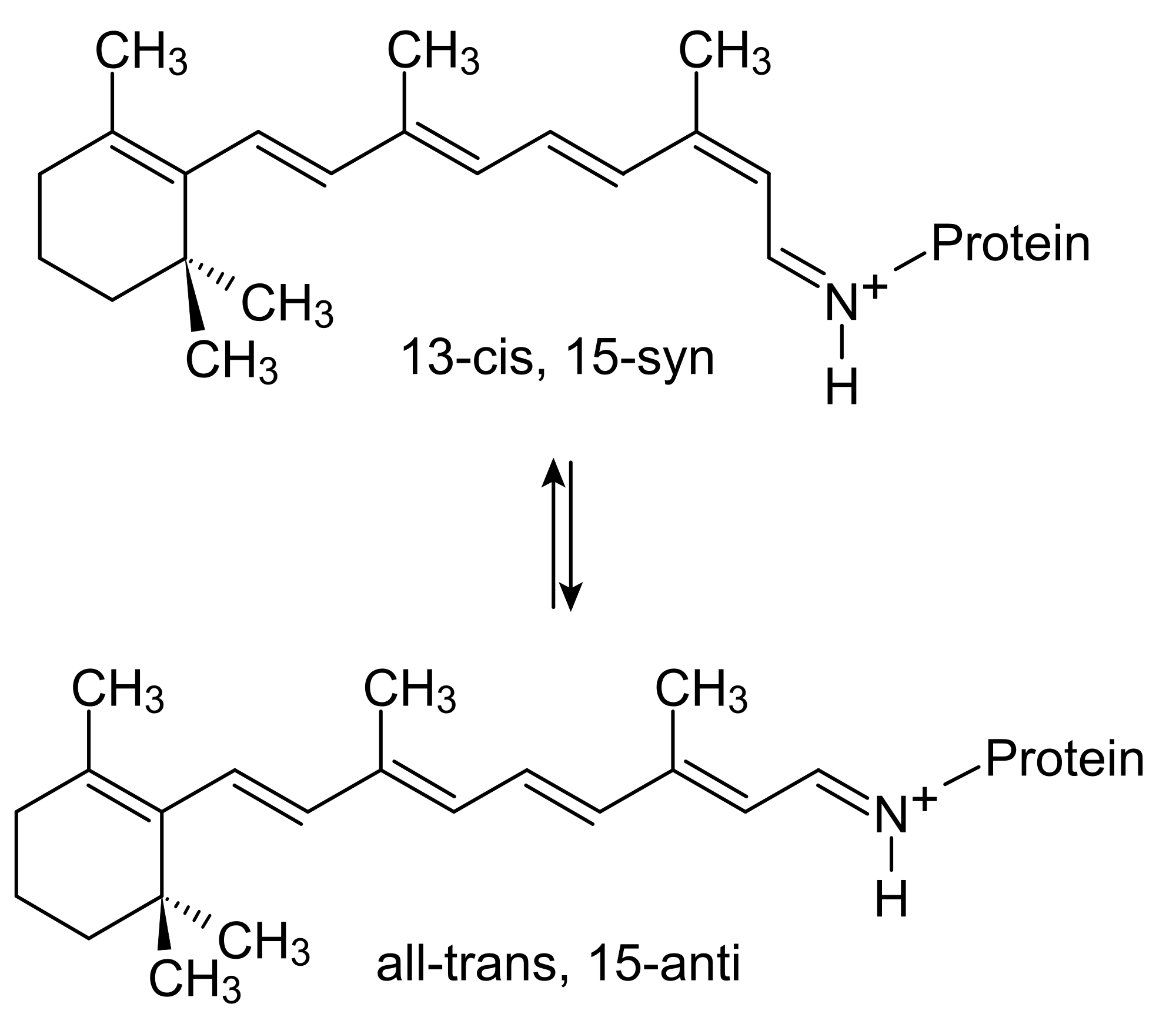
Bacteriorhodopsin
Bacteriorhodopsin is a protein mechanism made by some archaea. It is a brilliant mechanism that uses retinal, a chromophore, to absorb light. Retinal absorbs light to isomerize, which is a change in the chemical structure. This creates a movement that the opsin protein regulates and controls. Bacteriorhodopsin acts as a proton pump.
The retinal group photo-isomerizes into 13-cis-retinal which releases a hydrogen ion. It then returns to its original trans isomer, which is its low energy state, by uptaking a hydrogen ion. Then, it can use light to break off a hydrogen all over again. The genius of this mechanism is that the protein is bound to the cell plasma membrane, and orients the retinal to release protons, or hydrogen atoms, outside the cell. The proton pump creates a negative pressure inside the cell, allowing other mechanisms to be triggered by the osmotic motion, like ATP producing structures.
Bleaching and Photodegradation
We know that electrons are the keys to how atoms respond to light. Electrons form the bonds between atoms in a molecule. So, if we change the positions or energy states of these electrons, we can also sever or change atom bonds. Using high energy light, like ultraviolet light, most organic color compounds can be broken. Try this yourself, see what leaving something in the sun will do to organic pigments. However, the thing about this is I could see it being a great way to detect gamma radiation. I could see us engineering a protein that is meant to degrade in response to gamma rays. Then have an enzyme or other compound interact with the degradation products.
This is a lot of what we have been thinking about when trying to formulate an actual plan on making the color change mechanism for our cats. These are also very interesting gateways to understanding molecular processes. I hope you are thinking along with us, and leave your comments, or questions in the section below.